Research
My research interest is centered around the functional dissection of sensory-to-motor transformation, combining developmental and evolutionary perspectives. Below is a brief description of my current/past projects.
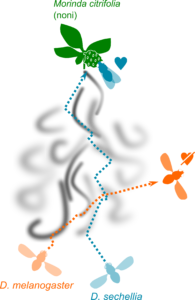
Evolution of olfactory circuits in drosophilids
Neuroscientists, including myself, strive to learn the general principles on how the brain works, often using model organisms. However, model organisms are distantly related to each other as well as to humans, making it difficult to translate perspectives across species. I believe that comparative, cross-species approaches to elucidate how nervous systems adapt to confer animals with unique behaviors will be an effective complementary way to extract general principles of brain organization and function.
In this project, we address the question of “How does sensory neuron number matter?” by leveraging the comparative framework. Sensory neuron number increase has been assumed to sensitize the central response and behavior, but this idea had not been experimentally tested. We compare the homologous olfactory circuits between Drosophila melanogaster and its close relative Drosophila sechellia, which underwent expansion of olfactory sensory neuron population detecting ecologically relevant cues. We combined genetic, molecular, neuroanatomical, neurophysiological, and neuroethological approaches and found out that sensory neuron number increase does not sensitize the central responses, in contrast to the long-held assumption. Rather, we found that it modulates olfactory habituation and enhances odor tracking behavior.
For details, see the following publication:
Takagi S†*, Sancer G, Abuin L, Stupski SD, Arguello JR, Prieto-Godino LL, Stern DL, Cruchet S, Álvarez-Ocaña R, Wienecke CFR, van Breugel F, Jeanne JM, Auer TO†*, Benton R* (†: equal contribution, *: corresponding authors) “Olfactory sensory neuron population expansions influence projection neuron adaptation and enhance odour tracking”Nature Communications, 2024, Aug 15;15(1):7041. doi: 10.1038/s41467-024-50808-w PMID: 39147786

This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 836783.
This work was also supported by EMBO Long-Term Fellowships (ALTF 454-2019).
Wiring and miswiring of sensorimotor circuits in Drosophila larvae
Adaptive behavior of an animal is underpinned by the complex yet precise connectivity of its nervous system. The connectivity of a neuron within the network is established through precise neurite guidance and synapse formation mechanisms, which give rise to the unique shape of individual neurons. How does this “shape” code behavior?
In this project, I study the molecular mechanisms underlying the segment-specific axon guidance of Wave neurons (see below) and the effect of their manipulation on behavioral regulation. We found that manipulation of one gene in Wave neurons suffices to rewire the tactile circuit and induce different behavior in response to tactile inputs.
For details, see the following publication:
Takagi S, Takano S, Hashimoto Y, Morise S, Zeng X, Nose A “Segment-Specific Axon Guidance by Wnt/Fz Signaling Diversifies Motor Commands in Drosophila Larvae” eLife, 2024 13:RP98624 doi: 10.7554/eLife.98624
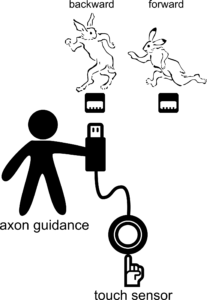
Segment-specific sensorimotor processing in Drosophila larvae
Sensory inputs of the same modality often elicit distinct behaviors depending on the location thereof. For instance, many animal species perform backward movement in response to head touch but perform forward movement to tail touch. How is the location-specificity realized in the brain? We showed that, in fruit fly Drosophila larvae, a class of segmentally-repeated interneurons (named Wave neurons) match their tactile receptive fields to appropriate motor programs by participating in different circuits in different segments.
For details, see the following publication:
Takagi S, Cocanougher BT, Niki S, Miyamoto D, Kohsaka H, Kazama H, Fetter RD, Truman JW, Zlatic M, Cardona A, Nose A. Divergent Connectivity of Homologous Command-like Neurons Mediates Segment-Specific Touch Responses in Drosophila. Neuron. 2017 Dec 20;96(6):1373-1387.e6. doi: 10.1016/j.neuron.2017.10.030. Epub 2017 Nov 30. PMID: 29198754.
